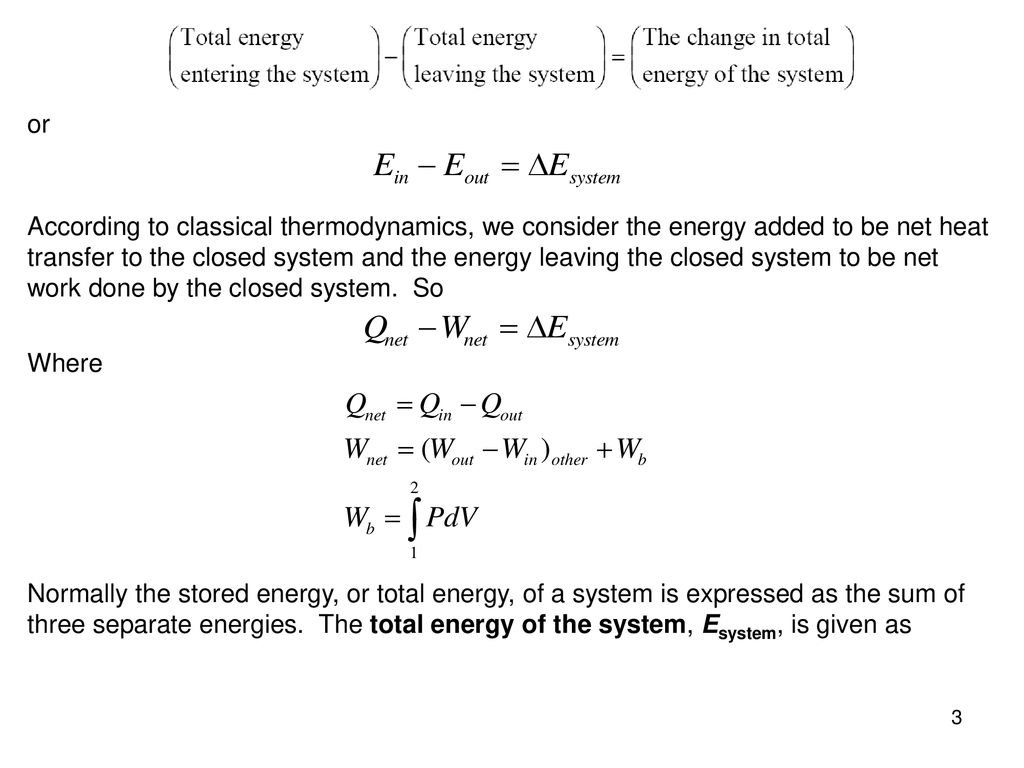
What Happens To Energy In An Open System
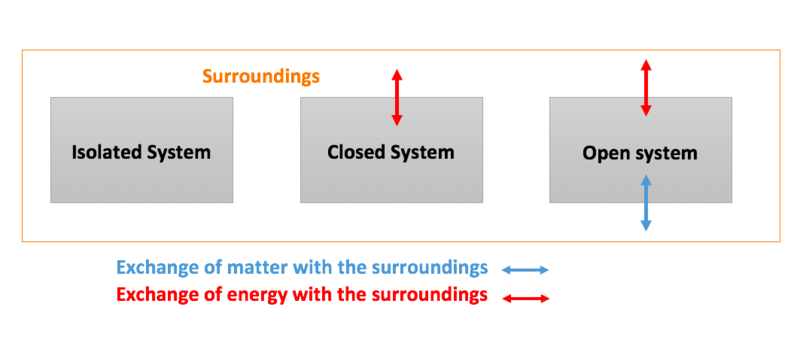
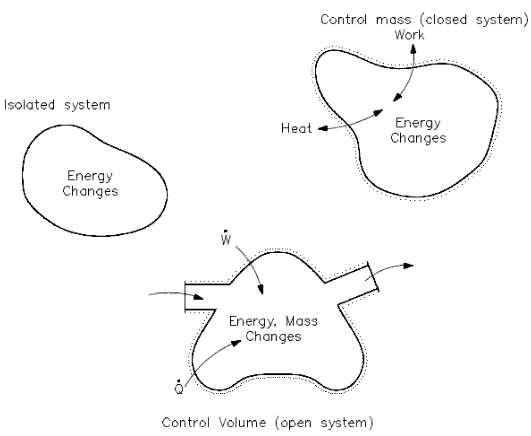
What happens to energy in an open system. An open system can exchange both energy and matter with its surroundings. The total energy is said to be conserved over time. When there are energy transfers in.
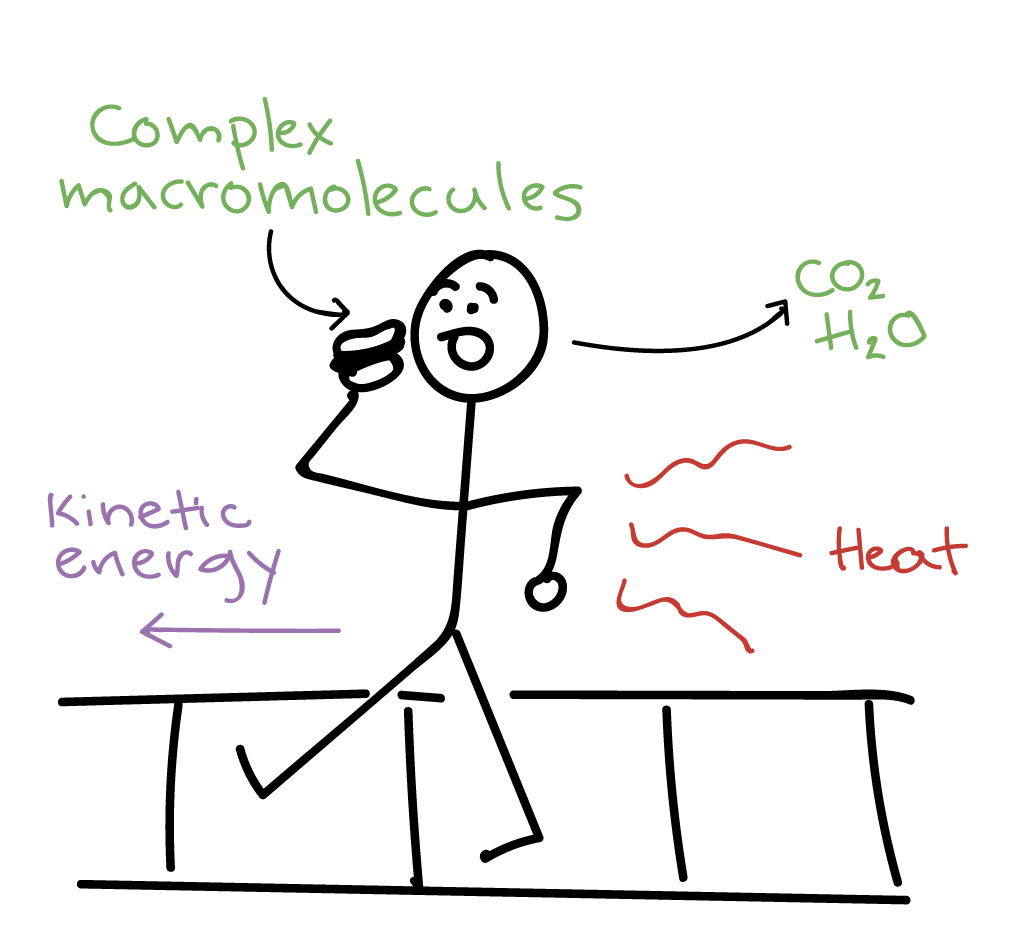
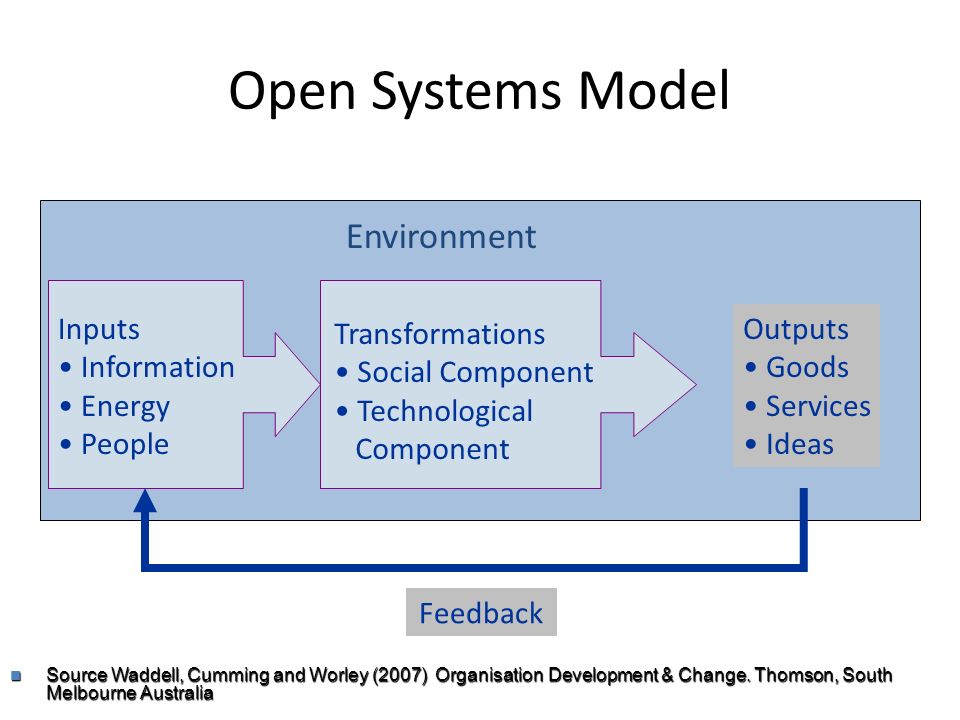
The total energy of a body in a conservative fieldKinetic energyPotential Energy. Energy is exchanged between them and their surroundings as they consume energy-storing molecules and release energy to. Energy can enter or exit an open system.
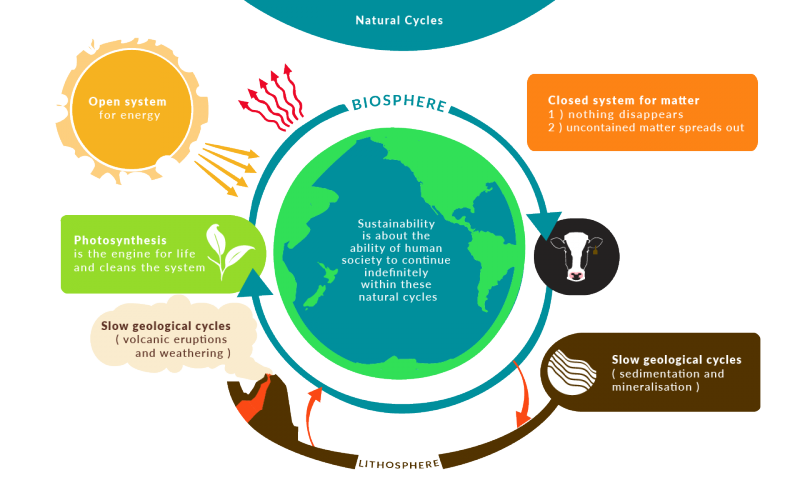
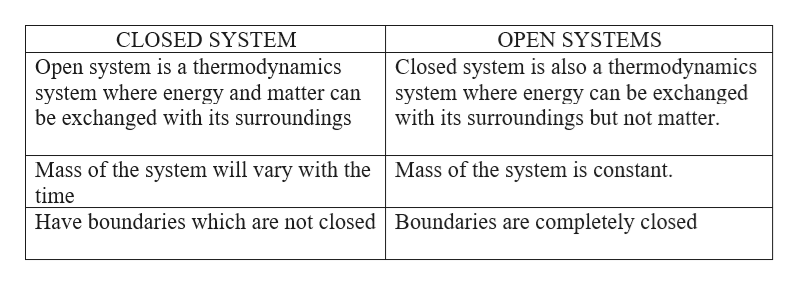
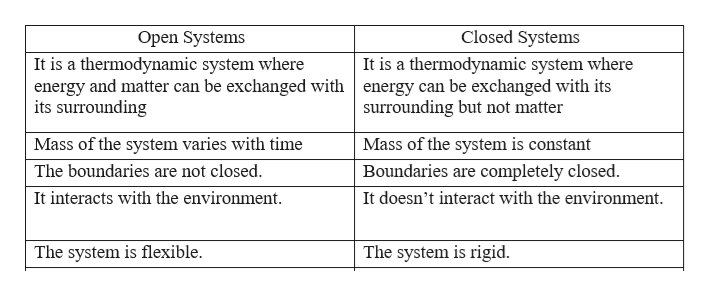
The main relations are those established between energy flows and nutrient flows. A closed system on the other hand can exchange only. What happens to energy in an open system.
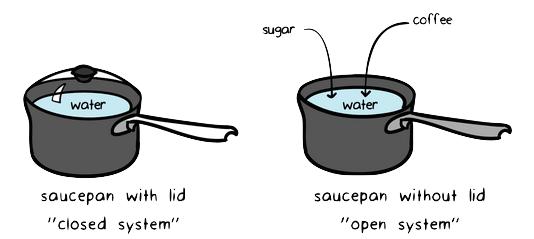
Explain the difference between an open system a closed system. The stovetop example would be an open system because heat and water vapor can be lost to the air. Bringing water to a boil on a gas hob.
Energy cannot enter or exit an open system. Energy from the chemical store in the gas increases the internal thermal energy of the water in the pan. Closed and Open Systems.
An open system is one in which energy can be transferred between the system and its surroundings. The stovetop system is open because heat can be lost into the air. Correct answer - What happens te energy in an open system A Energy can ex but not enter an open system B Energy cannot enter or exit an open system 6.
They create structures of greater internal energy ie they lower entropy out of the nutrients they absorb. Yes it can.
What happens to energy in an open system.
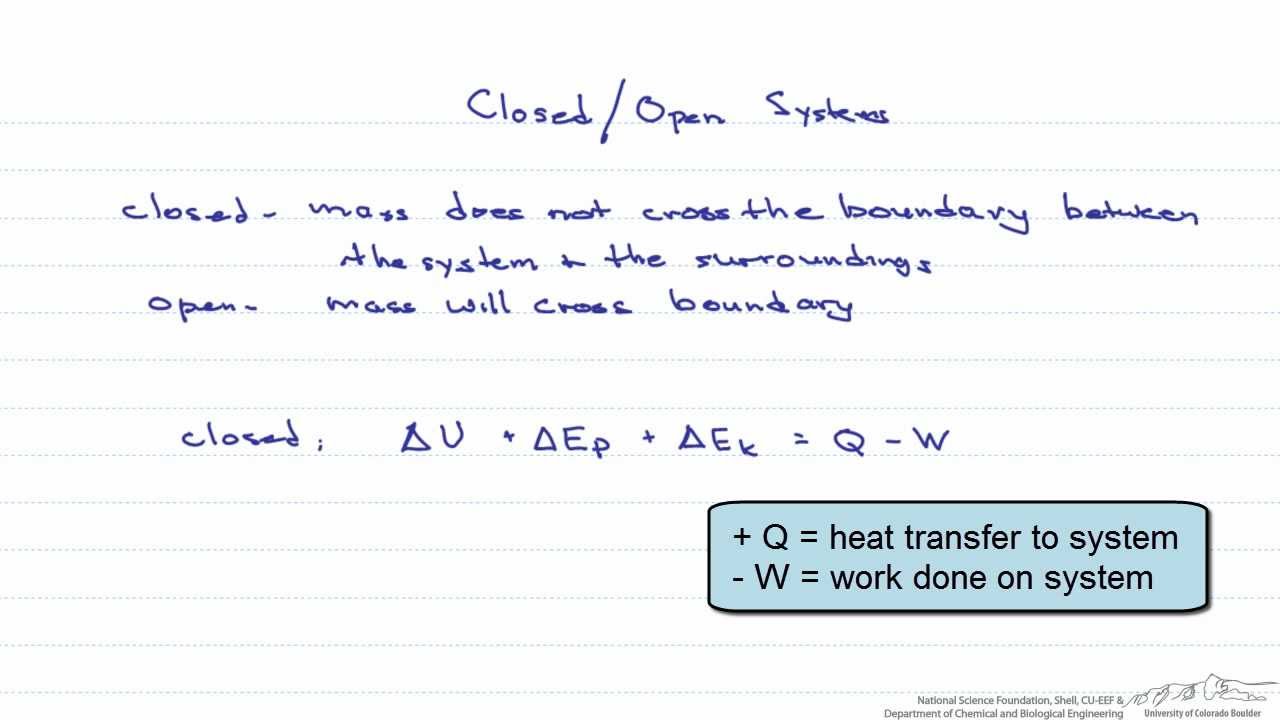
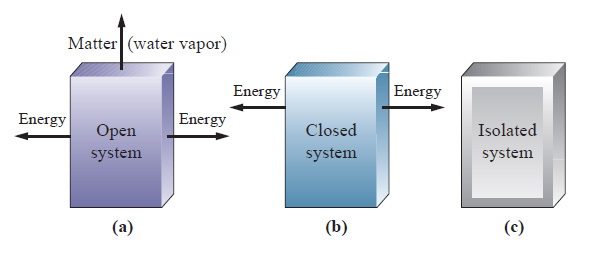
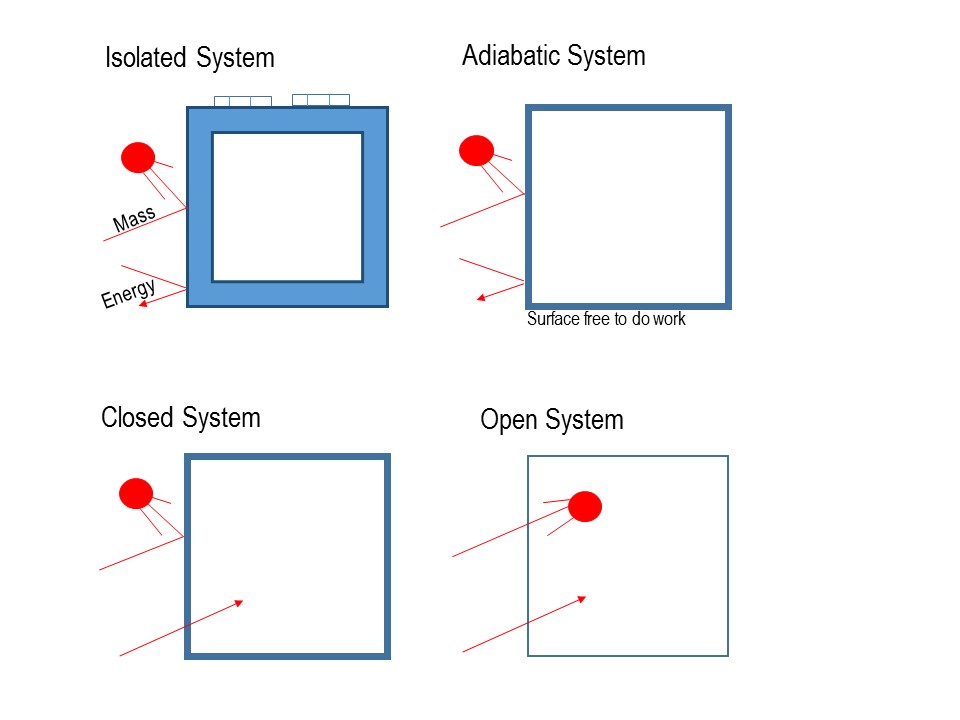
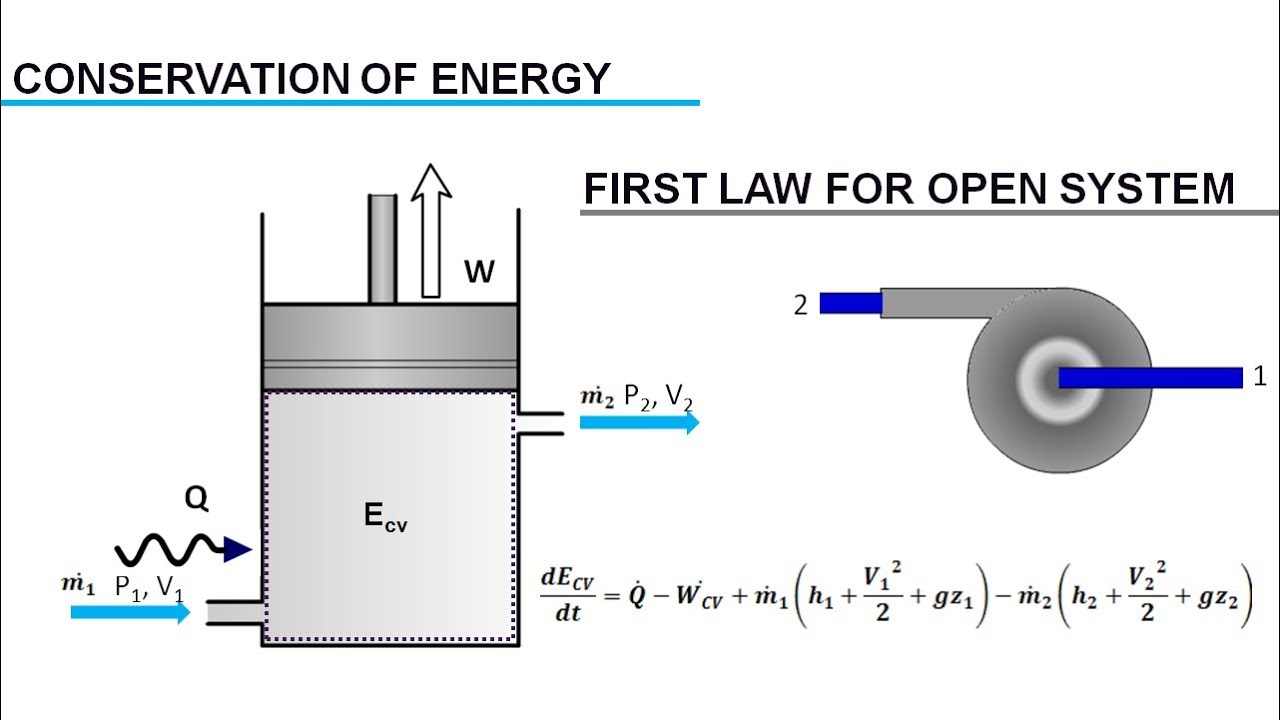
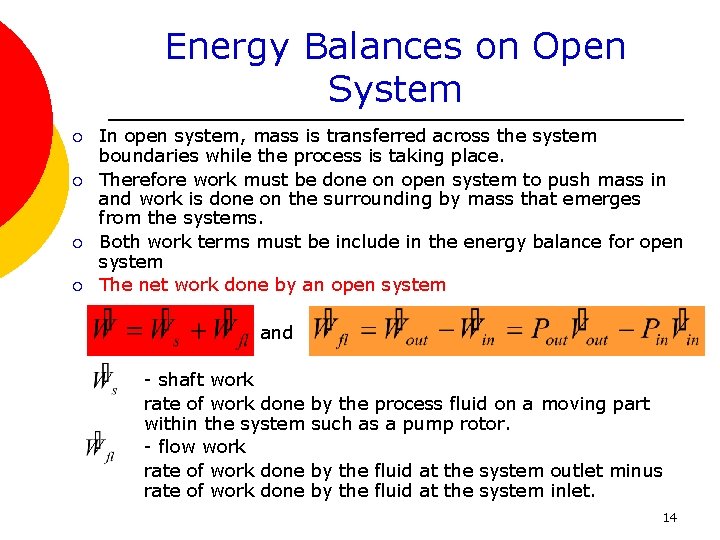
What happens to energy in an open system. Closed- unable to exchange energy or matter with surroundings open- energy and matter can be transferred between system and surroundings organisms are open systems. What happens to energy in an open system. Energy cannot enter or exit an open system. Here is a quick review of mass and energy balances for open and closed systems. Correct answer - What happens te energy in an open system A Energy can ex but not enter an open system B Energy cannot enter or exit an open system 6. Energy can enter or exit an open system. The stovetop system is open because heat can be lost into the air. Energy can enter but not exit an open system.
A closed system on the other hand can exchange only. 524 U u n i. The relations between the different components of an ecosystem are so close that if one of them is damaged the whole ecosystem is affected. The total energy of a body in a conservative fieldKinetic energyPotential Energy. The stovetop example would be an open system because heat and water vapor can be lost to the air. They create structures of greater internal energy ie they lower entropy out of the nutrients they absorb. An open system can exchange both energy and matter with itssurroundingswhere as a closed system CAN exchange energy but CANNOTexchange matter with its surroundings.























Post a Comment for "What Happens To Energy In An Open System"